Every patient’s cancer is unique
Cancer is a heterogeneous disease, yet treatment guidelines focus on one mutation at a time and do not consider mutational interactions and mechanisms of action that often cause drug resistance. The number of FDA approved cancer drugs has skyrocketed in the past decade, complicating the selection of the best treatment for individual patients.
Personalized Oncology through Therapy Biosimulation
Cellworks Therapy Biosimulation Reports
Cellworks provides a decision support system for oncologists challenged with determining the best treatment for treatment-naive as well as relapsed or refractory patients. Cellworks provides two products to assist in treating patients diagnosed with specific oncology indications.
SINGULATM Standard Care Response:
Cellworks SingulaTM reports provide personalized response predictions to Standard Care therapies for front-line patients.
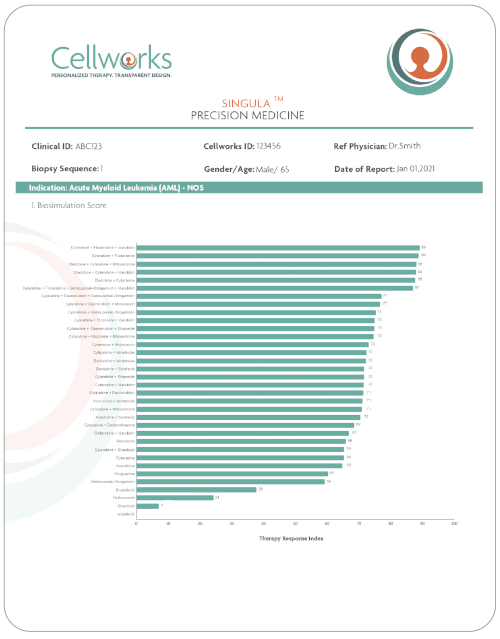
The SingulaTM report provides a Therapy Response Index (TRI) Score for every Standard Care drug for a given indication, ranking the impact of each therapy on the phenotype of a patient.
This enables physicians to know the benefit from treatment prior to treatment, preventing unwanted rounds of therapy that would be ineffective.
VENTURATM Ideal Combination:
Cellworks VenturaTM reports provide personalized therapy response predictions to combinations of FDA-approved drugs, including off-label drugs, for refractory patients.
The Cellworks Platform runs exhaustive combinations of drugs from Cellworks drug library to rank the most efficacious treatments for a patient.
This provides physicians treatment options beyond Standard Care for treatment-refractory patients, for whom there is little guidance on treatment.
Therapy Response Index (TRI)
Cellworks biosimulation reports use a Therapy Response Index (TRI) to score how effective therapies will be prior to treatment. Each therapy and combination of therapies is scored from 1 to 100 based on the patient’s unique biological response.