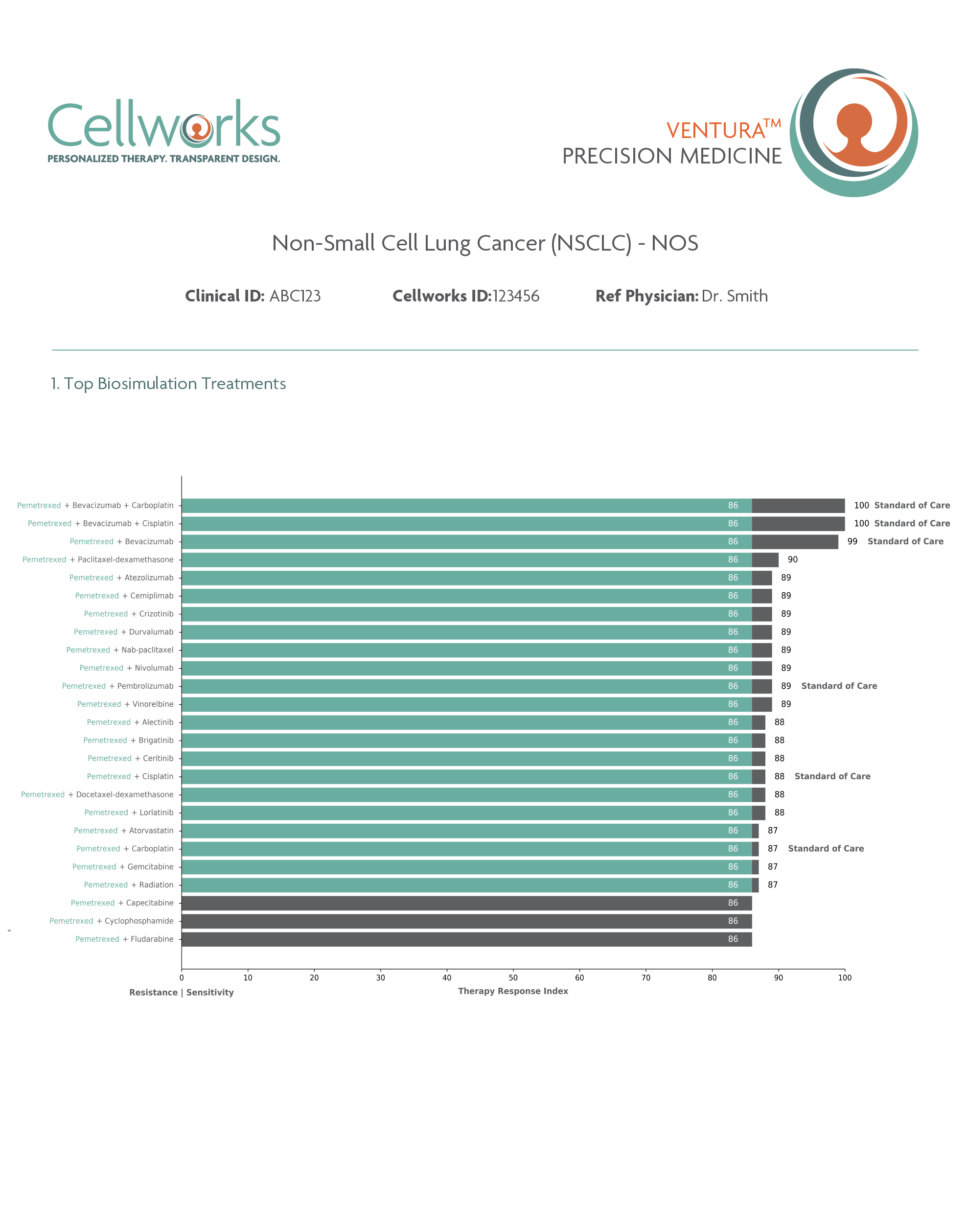
Cellworks Singula™ Therapy Response Index (TRI) Identifies Superior OS Outcomes for NSCLC Patients: myCare-203A
2021 : Computational Omics Biology Model (CBM) Identifies Novel Biomarkers to Inform Combination Platinum Compound Therapy in NSCLC
2021 : Impact of KRAS and Cooccurring Mutations on NSCLC Master Regulator Network as Determined by Computational Omics Biology Model
2021 : Computational Omics Biology Model (CBM) Identifies PD-L1 Immunotherapy Response Criteria Based on Genomic Signature of NSCLC

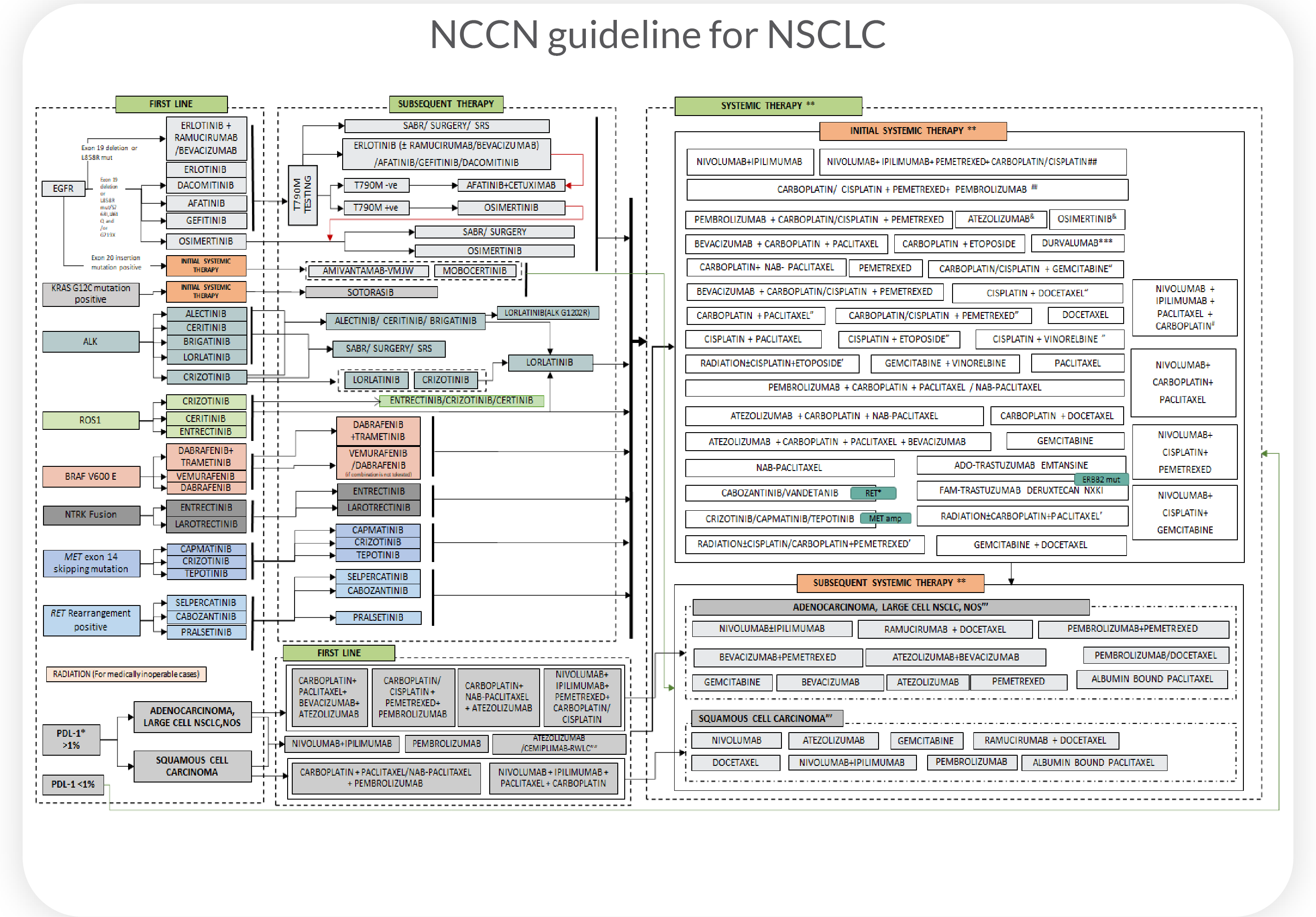
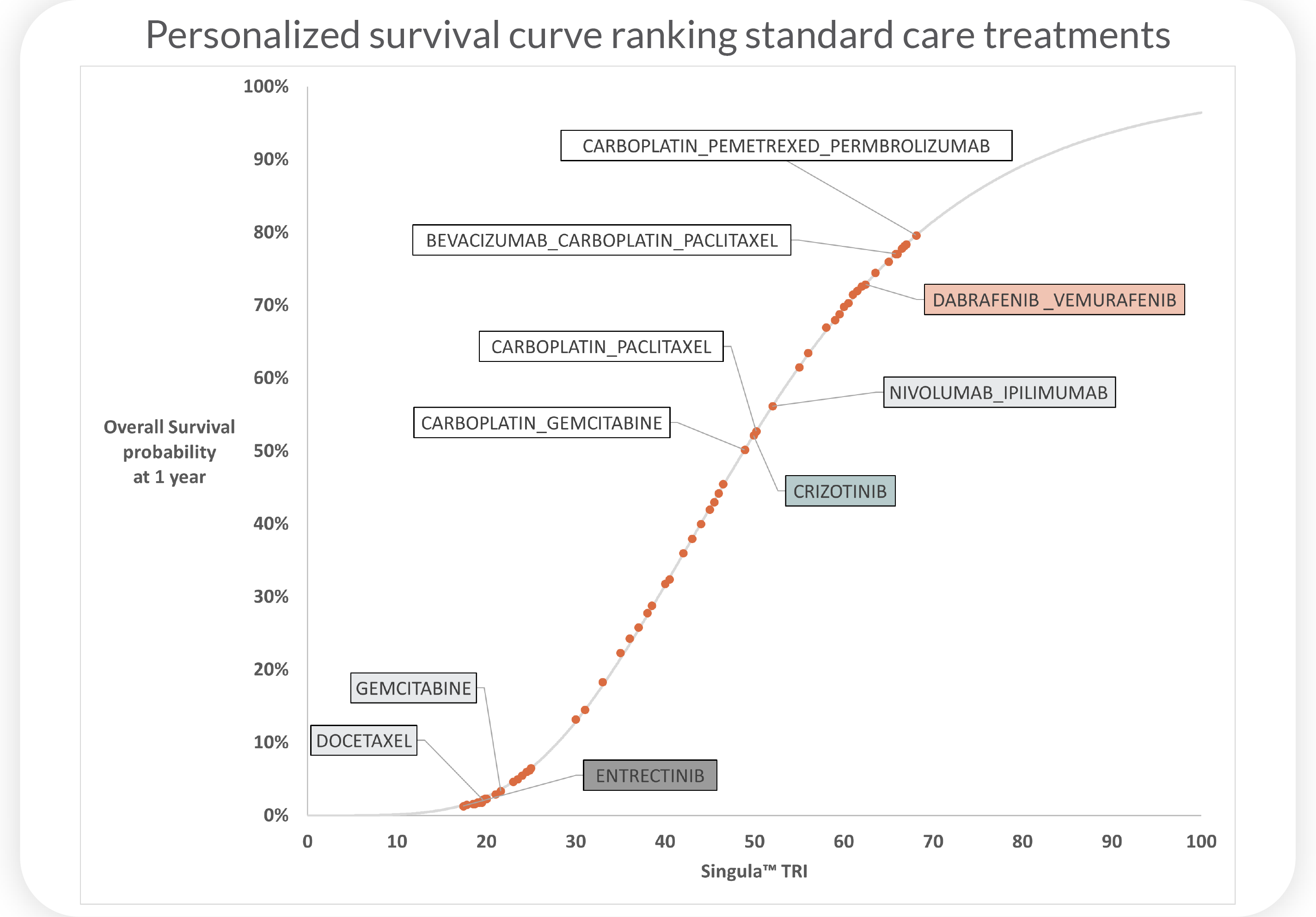
Superior Overall Survival(OS), progression-free survival(PFS), and clinical response(CR) predictions for patients with non-small cell lung cancer (NSCLC) using Cellworks Singula™ : myCare-022-05
to personalized therapy predictions
in 30 seconds

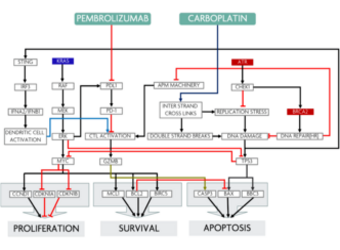
Predicting response to treatment with Carboplatin + Pembrolizumab in a NSCLC patient
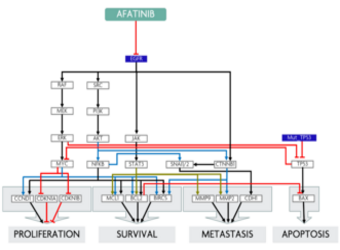
Predicting response to treatment with Afatinib in a NSCLC patient

Predicts personalized response to Standard Care therapies for front-line patients
Predicts and ranks personalized response to combinations of FDA-approved drugs including off-label and non-oncology drugs for refractory patients
Observational Clinical Trial for Assessment of Clinical Utility and Usability of Cellworks Reports
CANCER TYPE
Pan-cancer (150+ cancer indications)
PURPOSE
Turn a patient’s NGS report into personalized therapy predictions at no cost and provide usability feedback on Cellworks reports through a survey. No requirement to follow therapy predictions.
COLLABORATORS
Avera and University of Tennessee
Clinical Trial to Predict Optimal Personalized Treatment for Veterans with NSCLC
CANCER TYPE
NSCLC
PURPOSE
A prospective clinical trial to determine if Cellworks personalized therapy biosimulation more accurately predicts patient response to NSCLC Standard Care therapies than physician prescribed treatment.
COLLABORATORS
Veterans Administration and University of Nebraska