Synergistic Effects of Ruxolitinib and Sorafenib on JAK2-Mutant Myelofibrosis Predicted by Cellworks Model
Ruxolitinib is approved for myelofibrosis due to the occurrence of a high frequency of the JAK2 mutation. However, other mutations are also reported in this indication. The FDA has approved ruxolitinib, a targeted therapy for myelofibrosis, but the median overall survival for patients is still only 5-6 years. This demonstrates the need for a holistic approach that considers multiple mutations for myelofibrosis patients.
Our objective was to identify a novel drug combination for myelofibrosis (MF) patients that synergistically enhances the effect of ruxolitinib considering a comprehensive mutation profile, not based on only one or two mutations.
To identify combinations of other drugs to enhance the effect of ruxolitinib in MF, we:
Created a cancer disease model of a JAK2-V617F mutation bearing cell line for predictive simulation
Identified the key molecular disease characteristics of the JAK2-driven disease network post ideal disease induction
Simulated the FDA approved drug on ideal disease induction which represent the personalized disease profile
Validated the predictions in a JAK2 mutant cell line
The predictive biosimulation approach from Cellworks provides a representation of disease physiology incorporating signaling and metabolic networks with an integrated phenotype view. We modelled the JAK2-V617F expressing SET-2 cell lines based on its genomic aberration data. We then screened a library of FDA approved drugs on the SET-2 computational model in combination with ruxolitinib by assessing impact on disease specific biomarkers and phenotypes of proliferation, viability and apoptosis as end points.
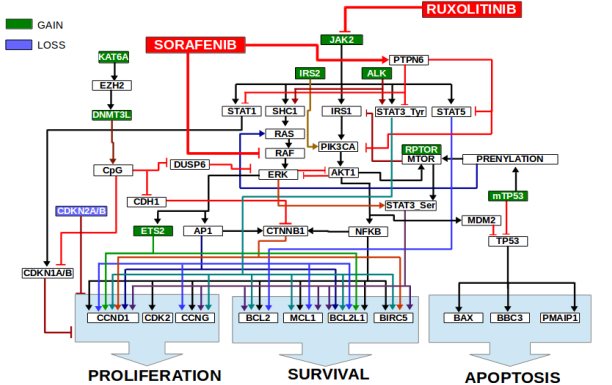
The modeling and digital drug screening identified a short list of drugs that were predicted to inhibit JAK2 signaling. Validation studies were conducted with the representative JAK2 driven MF model: SET2 cell line, which harbors the JAK2-V617F mutation and was predicted to lead to subsequent activation of STAT3, STAT5, ERK via SHC1, AKT via IRS1-PIK3CA and STAT1. JAK2 is known to phosphorylate STAT3 on tyrosine-705.
Predictive modeling also discovered activation of parallel signaling via mutations of ALK, IRS2 and RPTOR. ALK is also known to activate STAT3 via tyrosine-705 phosphorylation and ERK via SHC1. IRS2 leads to activation of PIK3CA-AKT-MTOR. SET-2 cells also harbor a RPTOR mutation which also activates MTOR pathway further. With higher ERK and MTOR signaling, there was serine-727 phosphorylation of STAT3.
Using digital drug screening on the SET2 simulation model, sorafenib mitigated the effect of JAK2 mutation by inhibiting pathways downstream of JAK2, rather than decreasing the JAK2 mutation burden itself. Sorafenib is a small molecule tyrosine kinase inhibitor that targets multiple proteins including VEGFR, PDGFR, and several Raf family kinases. It is approved for use in renal, liver, and thyroid cancers and is under clinical investigation for a number of other neoplasias.
A potential role for sorafenib in MF has not been fully investigated. Sorafenib is known to inhibit ERK via inhibition of RAF. Sorafenib was also predicted to inhibit STAT3, STAT5, STAT1 and AKT1 via activation of PTPN6 in the SET2 MF model. With down-regulation of ERK and AKT1-MTOR pathway, Sorafenib was predicted to inhibit STAT3 serine-727 phosphorylation. Hence, with sorafenib and ruxolitinib, not only was JAK2 burden reduced, but also parallel pathway signaling via ALK, IRS2 and RPTOR.
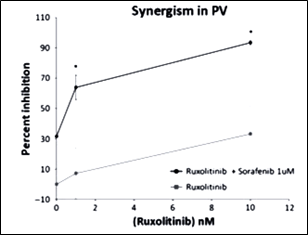
Using in vitro validation, we found that sorafenib was highly efficacious in reducing JAK2-V617F cell viability in a dose-dependent manner (GI50 = 6 uM). Moreover, when JAK2-V617F cells were treated with both ruxolitinib and sorafenib, there was synergy, suggesting that the compounds were acting on multiple, cooperating signaling pathways. A prior report also showed synergistic MPN regression with ruxolitinib and sorafenib in BaF3/JAK2-V617F and HEL cell lines and primary specimens, thereby providing independent evidence for the rationale use of these compounds in MF patients harboring mutation of PDGFRA/B, NRAS, KRAS in addition to JAK2 mutations.
Scientific Rationale:
Result:
In Conclusion
We demonstrated that successful treatment of MF likely requires targeting multiple, non-redundant, constitutively active, and cooperative signaling pathways, rather than a one-biomarker/one-drug approach as is the current clinical standard. Further, we showed how Cellworks predictive biosimulation modeling can be used for precision medicine purposes in MF to inform clinical treatment decision-making of FDA approved drugs, with the intent of providing significant health and cost benefits.




